徐贝贝同学于The Journal of Physical Chemistry Letters期刊发表文章
本实验室徐贝贝同学等在ACS旗下的《The Journal of Physical Chemistry Letters》期刊发表了题为《Solvent Water Controls Photocatalytic Methanol Reforming》的文章,通讯作者为王海丰老师,王雪璐老师和姚叶锋老师,发表日期为2020年4月21日。(DOI: 10.1021/acs.jpclett.0c00972)
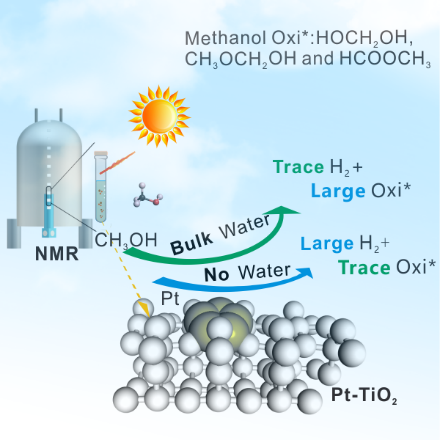
Understanding the role of different solvent molecules for practical solid-liquid heterogeneous photocatalytic reactions is critical to determine the pathway of the reaction. In this study, the operando nuclear magnetic resonance (NMR) method, combined with density functional theory (DFT) calculations, was employed to evaluate the control effect of solvent water in the photocatalytic reforming mechanism of methanol with a Pt-TiO2 catalyst. Results indicate that the presence of water effectively promotes the formation of the HCHO intermediate but inhibits the H2 evolution originating from the switch of the hydrogen source of the H2 formation from CH3OH to H2O. As detected directly in the ab-initio molecular dynamics (AIMD), a small amount of H2O can dissociate and the evolved -OH species at Ti5c site can greatly reduces the C-H activation barrier of -CH3O, which contributes to the formation of oxidation products (e.g., HOCH2OH and CH3OCH2OH) on Pt-TiO2 surface.
水作为地球上存在最广泛的液体,也是固液催化反应中常见的溶剂体系。我们利用原位液体核磁和动力学模拟等手段解释了溶剂水在光降解甲醇中扮演的双重身份,一方面以-OH构型显著降低甲醇的C-H裂解能垒生成大量甲二醇和半缩醛等氧化产物;另一方面,水分子和其裂解形态抢占表面活性位点同时具备高能垒难以被清除,明显抑制了甲醇产氢的过程,将氢气的质子源由甲醇分子切换为水分子。